International Journal of Agriculture, Environment and Biotechnology
Citation: IJAEB: 13(1): 105-110, March 2020
DOI: 10.30954/0974-1712.1.2020.14
©2020 IJAEB All rights reserved
AGRICULTURAL ECONOMICS
Studies on Induction of Somaclonal Variation in Sugarcane (Saccharum offcinarum) and Validation of Mutant Using Molecular Markers
Chinmay Gupta*, Sushma Nema, Swapnil Sapre and Keerti Tantwai
Biotechnology Centre, College of Agriculture, Jawaharlal Nehru Krishi Vishwa Vidyalaya, Jabalpur, M.P. India
*Corresponding author: chinmaygupta95@gmail.com ( ORCID ID: 0000-0002-6265-6174)
Paper No.15
Received: 14-09-2019
Revised: 03-01-2020
Accepted: 25-02-2020
ABSTRACT
Sugarcane is a perennial grass of the family Poaceae, primarily cultivated for its juice from which sugar is processed. Sugarcane yields are declining due to varietal degeneration and susceptibility to biotic and abiotic stresses. Smut of sugarcane is one of them, the disease can cause significant losses in cane tonnage and juice quality. As development of superior sugarcane varieties through conventional hybridization program is time consuming and has the problem of transfer of undesirable characters/traits into the newly developed hybrids/variety. In this connection, attempts were made to introduce genetic variability in sugarcane byin vitroculture techniques and mutation breeding. Induction of somaclonal variation is one of them which is described as the genetic variability present among cultured cells, plants derived from such cells or progeny of such plants. The treatment, MS medium supplemented with 3 mgl-1 2,4-D found most suitable for callus induction and multiplication. The callus on application of EMS become pinkish brown in colour, shoot regeneration from callus cells was high in MS medium supplemented with 3 mgl-1 BAP gave dark gray or brown and sticky callus. Molecular analysis of somaclonal mutants showed by primers polymorphism of DNA bands on validating with molecular markersviz.RAPD and ISSR markers gave 100 per cent polymorphism by two RAPD primers named OPA-01 and OPA-02 showed that out of 17 DNA samples of mutants considered, all were showing variations but one DNA sample was showing similarity with mother plant. The present investigation reports the study induction of somaclonal variation in Sugarcane (Saccharum offcinarum) and validation of mutant using molecular markers in variety Co261.
Highlights
 The Present study contains the process of induced mutation by the use of chemical mutagen EMS throughin- vitroculture of sugarcane variety Co261and while molecular analysis with RAPD and ISSR primers seventeen putative DNA samples were validated along with mother plant DNA which showed variations among them except mutant M5 which was showing similarity with mother plant Co261.
The Present study contains the process of induced mutation by the use of chemical mutagen EMS throughin- vitroculture of sugarcane variety Co261and while molecular analysis with RAPD and ISSR primers seventeen putative DNA samples were validated along with mother plant DNA which showed variations among them except mutant M5 which was showing similarity with mother plant Co261.
Keywords: Sugarcane, Somaclonal Variations, Induced Mutation, Molecular Markers
Sugarcane, (Saccharum offcinarum) is a perennial grass of the family Poaceae, which is primarily cultivated for its juice to obtain sugar. Most of the world’s sugarcane is grown in subtropical and tropical areas. However, five agro-climatic zones have been identified mainly for the purpose of varietal development. Sugarcane by-products serve as raw materials for the generation of paper pulp, plywood boards, animal feed, wax, biofertilizers, alcohols and many other useful products. Sugarcane yields are declining due to varietal degeneration and susceptibility to biotic and abiotic stresses. Whiptail Disease or Sugarcane Smut, Smut of sugarcane is caused by the fungusSporisorium scitaminaeis an important disease of sugarcane. The most recognizable diagnostic feature of sugarcane infected with smut is the emergence of a long, elongated whip. The whip morphology differs from short to long, twisted, multiple whips etc. The disease can cause significant losses in cane tonnage and juice quality. As development of superior sugarcane varieties through conventional hybridization program is time consuming and has the problem of transfer of undesirable characters/ traits into the newly developed hybrids/variety. In this connection, attempts were made to introduce genetic variability in sugarcane by in vitro culture techniques and mutation breeding (Begumet al.2011; Kumaret al.2012; Rastogiet al.2015; Shahidet al.2011; Yasmeenet al.2017.) Induction of somaclonal variation is one of them which is described as the genetic variability present among cultured cells, plants derived from such cells or progeny of such plants. Hence based on the above facts, the present investigation is meant for studies on induction of somaclonal variation in Sugarcane (Saccharum offcinarum) and validation of mutant using molecular markers for variety Co261.
MATERIALS AND METHODS
The commercial cultivar Co261 of sugarcane grown in Madhya Pradesh was employed as the source of explants. Healthy and disease-free sugarcane tops obtained from KVK Narsinghpur and from other sugarcane cultivating farmers of Narsinghpur, Madhya Pradesh, India.were used as explants.
In order to study the morphogenic response of sugarcane explants the most widely accepted MS medium (Murashige and Skoog 1962) was used as basal medium. The stock solutions were prepared fresh after every 4 to 6 weeks and that of the growth regulators were prepared fresh after every week. The supplement to be incorporated such as sucrose 30gl-1 into the basal medium were added before final adjustment of the volume prepared by double distilled water and further the plant growth regulators were added and then pH of the medium was adjusted at 5.8 ± 0.5 using either 0.1N NaOH or 0.1N HCl. The medium was solidified with bacteriological grade agar added before autoclaving at 120 ºC at 15 psi. Surface sterilization of explant leaf roll (sugarcane tops of about 10 cm) was done by 4-5 time washing under running tap water and then initially treated with treated with Tween-20 (5-7 drops) for 20 min proceeded by seventy percent of ethanol and different concentration of mercuric chloride for 3, 4 and 5 min respectively. Sterilized leaf roll explants were washed thoroughly with sterile distilled water for 5-7 times to eliminate the mercuric chloride in a laminar air- flow cabinet.
The segments of leaf roll were excised from contamination-free shoot apices of sugarcane variety Co261and cultured on MS medium supplemented with different levels of 2,4-D to know the response on callus induction and maintained by subculturing at every 21 days with 2,4-D (3.0 mg/l). After 60 days, the callus were transferred to callus induction medium containing 8 µM Ethyl methane sulfonate (EMS). After induction of somaclonal variation, callus were transferred to regeneration medium containing different concentrations of BAP. After shoot differentiation from established callus cultures DNA was isolated, purified, quantified and diluted PCR conditions were standardized considering different parameters viz. initial denaturation, denaturation, annealing, extension and final extension. PCR thermal and reaction profile was optimized for amplification purpose by using primers of unique sequence. PCR products mixed with gel loading dye was loaded on 1.5% agarose gel along with appropriate size DNA ladder (1kb) and run under constant voltage of 70 volt. The gels were visualized under UV light in gel documentation system and photographed through the same. DNA fingerprints on ISSR and RAPD gels were scored for the presence (1) or absence (0) of bands of molecular weight size in the form of binary matrix for all the accessions studied using DARwin6 Software. A dendogram was constructed using UPGMA (un weighted pair-group method with arithmetic averages) with the SAHN (sequential, agglomerative, hierarchical, and nested clustering) routine.
RESULTS AND DISCUSSION
MS medium fortified with different concentrations of 2,4-D were used for callus induction were incubated at 25±2°C with photosynthetic photon of 50 µmol-2s-1 under 16h/8h photo period. Callus induction was observed only on MS medium supplemented with 2,4-D alone. Callus of loose and fragile nature was observed on plumule region after 10-12 days of incubation which further spread throughout the seedling. On Maximum callus induction (52.22%) was obtained on MS medium fortifed with 3 mg/l 2,4-D (Plate 2 B and D) followed by 47% callus induction on MS medium fortified with 4mg/l 2,4-D (Plate 2 A and C ). With the increase in concentration from 1 mg/l to 4 mg/l of 2,4-D into MS medium, callus induction gradually enhanced in mature leaf roll explants. It is evident from the results that concentrations of auxin significantly affected both the traits. These results are in consonance with Kale (2004). According to Lal et al. (1992), in fast growing callus DNA, RNA and protein content increase rapidly and the condition becomes different during callus growth.
Table 1: Callus induction from leaf roll explants
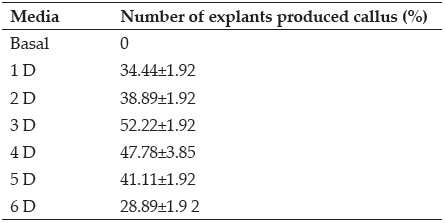
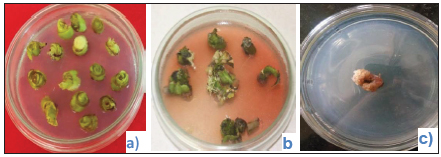
Fig. 1: Emergence of callus in 2,4-D(a) to(b) and effect of EMS on callus cultures(c) Callus after application of EMS
Calli were treated with Ethyl methane sulfonate (EMS) in order to increase the mutation frequency and enhance somaclonal variation after 60 days, the callus were transferred to callus induction medium containing 8 µM EMS. Studies have shown that EMS induces mutations (Hoffmann et al. 2004), thus generating variation amongst plants produced from the treated calli. Further, several studies have reported on EMS treatments to calli and subsequent selection of plants with desired traits using appropriate selection protocols. These traits include herbicide resistance, salt tolerance and disease resistance (Matsumoto et al. 2010).
After callus induction, calli were sub-cultured on shoot induction medium. Callus culture in shoot induction medium turned in embryogenic callus after 7 days and leaf primordia were induced within 15 days of culturing. These leaf primordia were elongated in the form of shoots within one month of culturing. MS medium supplemented with different concentrations of BAP, maximum shoot induction (55.75%) and shoots per callus (4.36) were obtained on MS medium fortified with 3 mg/l BAP followed by (48.85%) callus exhibiting shoot induction with (3.62) shoots on MS medium fortified with 2 mg/l BAP.

Fig. 2: Callus after application of EMS
Shoot induction response and numbers of shoots were significantly reduced with increasing concentration of BAP. MS medium fortified with BAP was found to be significantly superior in terms of both shoot induction percent and number of shoot per callus. Study done by Kumaret al.(2012) showed a large number of shoots were regenerated from such calli by subculturing on Murashige and Skoog’s medium containing BAP and Kinetin (0.5 mg/l each). These results clearly indicate that a lower concentration of cytokinin is required for shoot induction in sugarcane.
Regenerated Callus

Fig. 3: Regenerated Callus
Regenerated Shoots

Fig. 4: Regenerated Shoots
Table 2: Shoots from leaf roll derived callus culture
Molecular Validation of Mutants
Scoring of bands
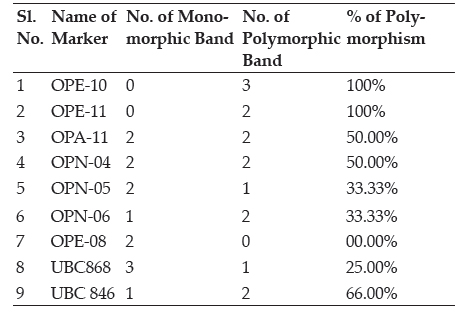
During the present study, initially 20 primers for RAPD and ISSR both were screened for amplifcation with two DNA samples from mixture of mutants A total of seven for RAPD and two for ISSR primers were amplified successfully and were selected on the basis of sharp and clear banding pattern for final RAPD and ISSR marker analysis. Out of seven RAPD primers OPE-10 and OPA-11 gave 100% of polymorphism while out of two ISSR primers UBC 846 gave 66% of polymorphism.
Table 3: Percentage of polymorphism score
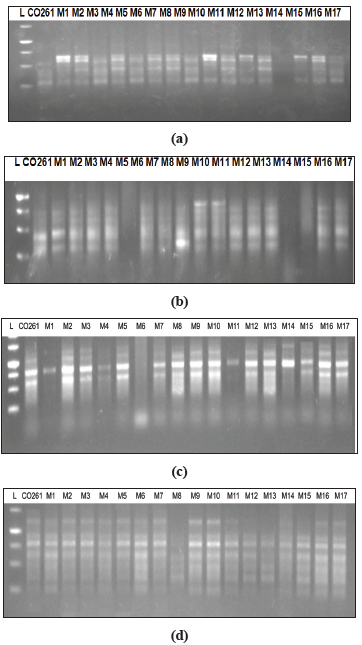
Fig. 5: Agarosegel electrophoresis of PCR amplified product with (a) & (b) RAPD and (c) & (d) ISSR
Analysis of mutants using molecular markers
Based on electrophoretic banding pattern of RAPD primers, pair wise genetic similarity among 17 putative samples of DNA were estimated and a dendrogram was generated. This analysis revealed that accessions of Sugarcane variety Co261 as a parent plant along with mutants obtained through somaclonal variation under study fell into two groups, major group (Group I) and minor group (Group II). Major group I divided into two sub groups, first subgroup ‘A’ which contained further two sub groups: Sub group ‘a’ which contained four mutantsviz.M 16, M6, M14, and M1 whereas sub group ‘b’ contained four mutants as well which are M13, M11, M7, and M8. Secondly subgroup ‘B’ contained mainly one mutant M5 along with mother plant Co261. Minor group II contained two sub groups first subgroup C which contained six mutantsviz.M17, M10, M12, M9, M3 and M2 whereas second sub group D contained only one mutant M15. It was notable that out of 17 DNA samples of mutants considered all were showing variations but one DNA sample was showing similarity with mother plant. Dalviet al.(2012) has screened out the promising sugarcane somaclones for agronomic traits and smut resistance using PCR amplification of Inter Transcribed Region (ITS) of Sporisorium scitaminae.
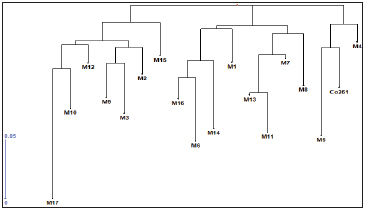
Fig. 6: Dendogram showing variations between mother plant of sugarcane variety Co261 and mutant obtained from it.
CONCLUSION
In vitroculture and induced mutagenesis are potentially-useful complementary approaches to conventional breeding for developing somaclonal variant plants exhibiting disease tolerance. The findings from the present study indicate that the treatment of calli (variety Co261) with EMS in combination with selection of tolerant calli. Indirect organogenesis clearly indicated its potential in inducing variability. Actually, genetic variation may have occured as a result of dedifferentiation of mature cells into callus stage, in the presence of plant growth regulators such as 2,4-D. Leaf roll proved as an better explants. Effective utilization of regeneration from callus may prove to be another way for creating some desirable variations with optimize conditions for increasing regeneration frequency. Plant tissue culture could be used in rejuvenating aging varieties like Co261 (Indian variety).
Suggestions for further work
1.Testing the stability of tolerance to mutant obtained in the current study in the lab, field and after sexual reproduction.
2.Assessing changes in smut resistance and levels of plant and fungal secondary metabolites in stalk tissue of Co261.
3.Testing the effect of somaclonal variation caused in Co261 for changes in agronomical traits.
4.Verifying the epigenetic characters of mutant.
ACKNOWLEDGEMENTS
I would like to take this opportunity to thank all the people who have helped me, in one way or another to gain this experience. Words cannot express my deepest core of my soul to my family whose selfless love constant and encouragement, sincere prayers to God, expectation and blessing have always been the most vital source of inspiration in my life.
Words are inadequate in the available lexicon to avouch the excellent guidance given by my major advisor Dr. Sushma Nema, Professor, Biotechnology Centre, JNKVV, Jabalpur and Dr. Amitava Rakshit Assistant Professor, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi, U.P. Their dedication to research, meticulous planning, consecutive consul and reserved help serve as a beacon light throughout the research work.
REFERENCES
Begum, M.K., Islam, M.O., Miah, M.A.S., Hossain, M.A. and Islam, N. 2011. Production of somaclone in vitrofor drought stress tolerant plantlet selection in sugarcane ( Saccharum offcinarumL.).The Agriculturists,9(1&2): 18-28.
Dalvi, S.G., Vasekar, V.C., Yadav, A., Tawar, P.N., Dixit, G.B., Prasad, D.T. and Deshmukh, R.B. 2012. Screening of the promising sugarcane somaclones for agronomic traits, and smut resistance using PCR amplification of Inter Transcribed Region (ITS) of Sporisorium scitaminae.Sugar Tech.,14(1): 68–75.
Hofmann, N.E., Raja, R., Nelson, R.L. and Korban, S.S. 2004. Mutagenesis of embryogenic cultures of soybean and detecting polymorphisms using RAPD markers.Biologia. Plantarum,48: 173-177.
Kale, P., Bruno, T.V. and Bhagade, S.V. 2004. Studies on callus initiation and plantlet regeneration in sugarcane ( Saccharumsp.)Indian J. Genet.,64(2): 165-166.
Khan, I.A., Seema, N., Raza, S.I. and Yasmin. 2015. Comparative performance of sugarcane somaclones and exotic germplasm under agro-climatic conditions of Tando Jam.Pakistan Journal of Botany,47(3): 1161-1166.
Kumar, P., Agarwal, A., Tiwari, A.K., Lal, M. and Jabri, M.R.A. 2012. Possibilities of development of red rot resistance in sugarcane through somaclonal variation.Sugar Tech.14(2): 192–194.
Lal, N., Chandra, P., Singh, J. and Singh, H.N. 1992. Changes in nucleic acid and protein contents during plant regeneration from callus in sugarcane.Indian J. Plant Physiol.,35(4): 389-392.
Mahlanza, T. 2012.In vitrogeneration of somaclonal variant plants of sugarcane ( Saccharumspp. hybrids) for tolerance to toxins produced by Fusarium sacchari. M.Sc. Thesis, University of Kwa Zulu-Natal, Durban, SA. pp. 156.
Matsumoto, K., Barbosa, M.L., Souza, L.A.C and Teixeira, J.B. 2010.In vitroselection for resistance to Fusariumwilt in Banana. In: Mass screening techniques for selecting crops resistant to disease. Joint FAO/IAEA Programme of Nuclear Techniques in Food and Agriculture. IAEA. pp. 101-114.
Patel, G.S. 2007.In vitroregeneration and detection of somaclonal variations inRauwolfia serpentine(L.) using molecular markers. M.Sc. Thesis, Jawaharlal Nehru Krishi Vishwa Vidyalaya, Jabalpur, pp. 62.
Rajeswari, S., Thirugnanakumar, S., Anandan, A. and Krishnamurthi, M. 2009. Somaclonal variation in sugarcane through tissue culture and evaluation for quantitative and quality traits. Euphytica., 168: 71–80.
Rastogi, J., Siddhant, Bubber, P. and Sharma, B.L. 2015. Somaclonal Variation: A new dimension for sugarcane improvement.GERF Bulletin of Biosciences,6(1): 5-10.
Shahid, M., Singh, A. and Shukla, P.K. 2011. Callus Induction in sugarcane genotypes.Trends in Biosciences,4(1): 21-22.
Smiullah, Khan, F.A., Abdullah, Afzal, A., Javed, M.A., Iqbal, Z., Ifikhar, R. and Watoo, J.I. 2012.In vitroregeneration, detection of somaclonal variation and screening for mosaic virus in sugarcane (Saccharum spp.) somaclones.South African Journal of Biotechnology,11(48): 10841-10850.
Yasmeen, S., Rajput, M.T., Khan, I.A. and Hasseny, S.S. 2017. Induced mutations and somaclonal variations in three sugarcane(Saccharum ofcinarumL. )varieties.Pakistan Journal of Botany,49(3): 955-964.
 The Present study contains the process of induced mutation by the use of chemical mutagen EMS throughin- vitroculture of sugarcane variety Co261and while molecular analysis with RAPD and ISSR primers seventeen putative DNA samples were validated along with mother plant DNA which showed variations among them except mutant M5 which was showing similarity with mother plant Co261.
The Present study contains the process of induced mutation by the use of chemical mutagen EMS throughin- vitroculture of sugarcane variety Co261and while molecular analysis with RAPD and ISSR primers seventeen putative DNA samples were validated along with mother plant DNA which showed variations among them except mutant M5 which was showing similarity with mother plant Co261.






